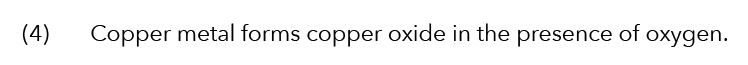Introduction
In this Secondary 2 Chemistry blog post, we’ll walk through a multiple-choice question on electrolysis, a concept that may seem tricky at first.
But don’t worry, we’ll break the word down and work through how you can use the meaning of the word to help you solve this question!
Alternatively, you can also watch our explainer video for free by visiting our YouTube channel!
Read Also:
Let’s Take A Look At This Chemistry Question
Source: Hougang Secondary School – 2017 S2NA SA2 Examination Paper [Q8]
The question wants us to choose which among the options best describes electrolysis.
Breaking Down The Term ‘Electrolysis’
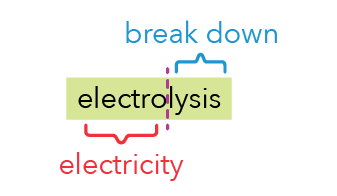
The word “electrolysis” can be split into two parts:
- Electro → suggests we are using electricity.
- Lysis → means that it splits apart things.
In more scientific terms, “lysis” means breaking down or decomposing.
To answer this question, we need to find the reaction where electricity is used to break something down.
Let’s Analyse Option (1)
“Broken down” is another word for decomposition.
When you see the word “heat” in a question, think of thermal decomposition, a reaction where a substance breaks down when heated.
Let’s Analyse Option (2)
This is the only option that mentions electricity and tells us that copper chloride is broken down.
This means that it is the correct answer, but let’s go through the remaining options to confirm our answer!
Let’s Analyse Option (3)
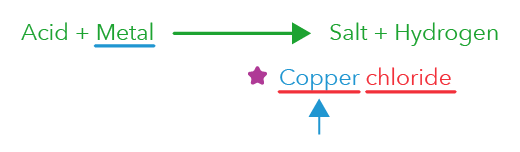
This is not an electrolysis reaction. This is an acid-metal reaction!
💡 Let’s Recap 💡
Acids react with metals to give us salt and hydrogen gas.
In this case, since the acid is hydrochloric acid, the salt name should end with chloride.
The first part of the salt name comes from the metal, which is copper.
So the salt should be copper chloride.
If you take a look at this option again, does it say that copper chloride is formed?
No, it says copper oxide, which is incorrect because acid-metal reaction, specifically when we react copper with hydrochloric acid, should get copper chloride as the salt.
Let’s Analyse Option (4)
What is the name of the reaction? This is simply oxidation.
But the question is asking us about electrolysis.
So this is also incorrect!
Suggested Answer
Option (2) is the correct answer ✅
It’s the only option that uses electricity to break down a substance, just like what electrolysis means!
Conclusion
I hope that after reading this blog post, you’ve learnt that when you come across unfamiliar terms in Science, you can try to break them down into smaller parts. This can make them easier to understand. Sometimes, the meaning of the term itself can even give you clues to help solve the question.
“Electrolysis” when broken down into 2 parts, becomes easier when you understand:
‘Electro’ means electricity, and ‘lysis’ means breaking down.
In this case, only Option (2) matches what we’re looking for: Electricity is used to break down a substance.
Stay tuned for more Chemistry blog posts!